FDA Approves Clinical Trial of Auditory Brainstem Implant Procedure for Children in U.S.
[Source: Science Daily]
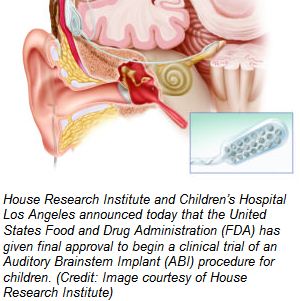
L.A.-based House Research Institute and Children’s Hospital Los Angeles announced today that the United States Food and Drug Administration (FDA) has given final approval to begin a clinical trial of an Auditory Brainstem Implant (ABI) procedure for children. The trial is a surgical collaboration sponsored by the House Research Institute in partnership with Children’s Hospital Los Angeles and Vittorio Colletti, MD of the University of Verona Hospital, Verona, Italy.
The ABI was developed at the House Research Institute and is the world’s first successful prosthetic hearing device to stimulate neurons directly at the human brainstem, bypassing the inner ear and hearing nerve entirely. Since the procedure began, more than 1,000 adults worldwide have received the ABI, with surgeons at the House Clinic leading the way.
Read the Rest of this Article on Science Daily.com
PediaStaff is Hiring!
All JobsPediaStaff hires pediatric and school-based professionals nationwide for contract assignments of 2 to 12 months. We also help clinics, hospitals, schools, and home health agencies to find and hire these professionals directly. We work with Speech-Language Pathologists, Occupational and Physical Therapists, School Psychologists, and others in pediatric therapy and education.
